When 1.50 mol of CH4(g) reacts with excess Cl2(g) at constant pressure according to the chemical equation shown below, 1062 kJ of heat are released. Calculate the value of ΔH for this reaction, as written.
2CH4(g) + 3Cl2(g) → 2CHCl3(l) + 3H2(g) 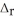 H° = ?
H° = ?
Definitions:
Equity Risk
The risk of loss associated with fluctuations in the price of equities or stocks.
M&M Proposition I
A principle in corporate finance that asserts the market value of a firm is unaffected by the capital structure, assuming no taxes and perfect markets.
M&M Proposition II
A theory proposing that the cost of equity increases with the level of debt in a company, making the firm's weighted average cost of capital remain unchanged.
Cost of Equity
The return rate that shareholders require to invest in a company's equity, taking into account the risk associated with the investment.
Q7: Identify the formula for barium hydroxide octahydrate.<br>A)
Q16: Identify the colour that has a wavelength
Q67: Using the periodic table, identify the element
Q78: How many millilitres of a 9.0 mol
Q97: Which of the following compounds is insoluble
Q108: Calculate the kinetic energy of a 150
Q114: Calculate the heat transfer (J) when 3.15
Q121: How many moles of BCl<sub>3</sub> are needed
Q200: What are the required coefficients to properly
Q203: A solution is prepared by adding 1.40