In the presence of excess oxygen, methane gas burns in a constant pressure system to yield carbon dioxide and water: 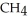 (g) +
(g) + 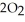 (g) →
(g) → 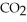 (g) +
(g) + 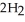 O (l)
O (l) 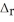 H = -890.0 kJ Calculate the value of q (kJ) in this exothermic reaction when 1.90 g of methane is combusted at constant pressure.
H = -890.0 kJ Calculate the value of q (kJ) in this exothermic reaction when 1.90 g of methane is combusted at constant pressure.
Definitions:
Q11: Describe the difference between complete ionic and
Q18: Identify the number of valence electrons for
Q33: Balance the following redox reaction if it
Q69: Calculate the amount (mass) of acetaldehyde (C<sub>2</sub>H<sub>4</sub>O,
Q92: What volume of benzene (C<sub>6</sub>H<sub>6</sub>, d =
Q115: Identify the net ionic equation for the
Q118: How many unpaired electrons are present in
Q125: Which of the following is FALSE?<br>A) Kinetic
Q134: Of the following, which atom has the
Q155: 5.0 g of iron is reacted with