How much energy is evolved during the formation of 98.7 g of Fe according to the reaction below?
Fe2O3(s) + 2Al(s) → Al2O3(s) + 2Fe(s) 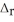 H° = -852 kJ
H° = -852 kJ
Definitions:
Lowest Terms
Lowest terms refer to a fraction that has been simplified to its smallest possible integer numerator and denominator.
Fractions
Fractions represent parts of a whole or any number of equal parts, typically written with a numerator and a denominator, separated by a slash.
Acres
A unit of land area measurement used in the U.S. customary and imperial systems, equal to 43,560 square feet or about 4,047 square meters.
Oats
A cereal grain known for its edible seeds, often consumed as oatmeal or rolled oats, and used as livestock feed as well as in various food products.
Q26: Using Lewis structures and formal charge, which
Q38: What is the volume of 9.783 ×
Q53: Place the following types of electromagnetic radiation
Q82: What was the predicted formula for the
Q113: Identify the gas that is the lowest
Q136: Se-I<br>A)strongest covalent bond<br>B)longest covalent bond<br>C)weakest ionic bond<br>D)metallic
Q159: How many moles of CuO can be
Q171: Automotive air bags inflate when sodium azide
Q197: Which of the following statements is TRUE?<br>A)
Q258: What is the concentration of nitrate ions