Using the following thermochemical equation, determine the amount of heat produced per kg of CO2 formed during the combustion of benzene (C6H6) . 2C6H6(l) + 15O2(g) → 12CO2(g) + 6H2O(g) 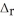 H° = -6278 kJ
H° = -6278 kJ
Definitions:
Feedback
Information or opinions about something, used as a basis for improvement or adjustment.
Backchannel
A secondary or covert route of communication used for private conversation or feedback during a public exchange.
Electronic Communication
The transmission of information using electronic means such as email, instant messaging, social media, and websites.
Nonverbal Cues
Communication signals that are not spoken or written but are expressed through body language, facial expressions, and other physical behaviors.
Q8: Determine the concentration of a solution prepared
Q10: A single covalent bond contains _ of
Q86: Calculate the temperature if 3.120 moles of
Q87: Determine the specific heat capacity of an
Q87: Which of the following quantum numbers describes
Q91: Identify the oxidation state of Mg in
Q121: Each of the following sets of quantum
Q150: Identify the number of valence electrons for
Q181: Identify the polyprotic acid.<br>A) H<sub>2</sub>SO<sub>4</sub><br>B) HCl<br>C) NaCl<br>D)
Q210: Dinitrogen monoxide gas decomposes to form nitrogen