Using the following equation for the combustion of octane, calculate the amount of grams of carbon dioxide formed from 100.0 g of octane. The molar mass of octane is 114.33 g mol-1. The molar mass of carbon dioxide is 44.0095 g mol-1. 2C8H18 + 25O2 → 16CO2 + 18H2O 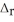 H° = -11018 kJ
H° = -11018 kJ
Definitions:
Maintenance Strategy
A plan for managing and conducting regular upkeep to ensure the optimal functioning and prolong the lifespan of assets or systems.
Monitoring Phase
A stage in various processes (e.g., treatment, project management) where progress is closely observed and assessed to ensure objectives are being met.
Support Systems
Networks of individuals or organizations that provide emotional, informational, and practical assistance.
Suicidal Ideation
The contemplation or consideration of ending one's life, which can range from fleeting thoughts to detailed planning.
Q18: Identify the correct values for a 2p
Q32: The action of some commercial drain cleaners
Q52: Choose the best Lewis structure for NO<sub>3</sub>⁻.<br>A)
Q84: Metallic aluminum reacts with MnO<sub>2</sub> at elevated
Q116: Calculate the enthalpy of solution for the
Q124: An element that has the valence electron
Q127: Why are the standard heats of formation
Q130: How are electron affinity and electronegativity different?
Q152: Convert 4744.1 mmHg to bar.<br>A) 7.549 bar<br>B)
Q223: A student prepared a stock solution by