A 100.0 mL sample of 0.300 mol L-1 NaOH is mixed with a 100.0 mL sample of 0.300 mol L-1 HNO3 in a coffee cup calorimeter. If both solutions were initially at 35.00 °C and the temperature of the resulting solution was recorded as 37.00 °C, determine the 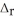 H° (in units of kJ
H° (in units of kJ 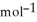 ) for the neutralization reaction between aqueous NaOH and HNO3 . Assume 1) that no heat is lost to the calorimeter or the surroundings, and 2) that the density and the heat capacity of the resulting solution are the same as water.
) for the neutralization reaction between aqueous NaOH and HNO3 . Assume 1) that no heat is lost to the calorimeter or the surroundings, and 2) that the density and the heat capacity of the resulting solution are the same as water.
Definitions:
Atmosphere
The layer of gases surrounding a planet, crucial for supporting life on Earth, composed mainly of nitrogen, oxygen, argon, and carbon dioxide.
Fossil Fuels
Naturally occurring carbon-based fuels derived from the decomposed remains of ancient plants and animals, such as coal, oil, and natural gas.
Energy Pyramid
For an ecosystem, a graphic depicting the energy that flows through each trophic level in a given interval.
Trophic Level
The rank in the food chain of an organism, determined by the number of energy-transfer steps to that level.
Q6: What is the effective nuclear charge experienced
Q14: energy associated with the motion of an
Q15: -w<br>A)energy flows out of the system into
Q20: Give an example of a d orbital.
Q24: Explain the difference between ΔH and ΔU.
Q59: A container filled with gas is connected
Q77: Use the bond energies provided to estimate
Q132: When silver nitrate reacts with barium chloride,
Q136: Identify the colour of a flame test
Q239: In an acid-base neutralization reaction, 38.74 mL