9.26 g of an unknown solid is dissolved in a coffee cup calorimeter that contains 62.0 mL of water. The measured temperature increased from 23.4 °C to 24.7 °C. The specific heat of water is 4.184 J g-1 °C-1, and assume the density of the solution is 1.00 g mL-1. Calculate the molecular weight of the unknown solid if the 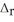 H was determined to be -2.51 kJ mol-1 via another experiment.
H was determined to be -2.51 kJ mol-1 via another experiment.
Definitions:
Community Health
A field of public health that focuses on promoting, protecting, and improving the health status of people in community settings.
Semicritical Items
Objects that come into contact with mucous membranes or non-intact skin and require a high level of disinfection to prevent infection.
Disinfection
The process of eliminating or reducing harmful microorganisms from inanimate objects and surfaces, usually through chemicals or heat.
Gastrointestinal Endoscopes
Specialized tools used for visual examination of the gastrointestinal tract, including the esophagus, stomach, and intestines, to diagnose and sometimes treat conditions.
Q14: energy associated with the motion of an
Q41: Which of the following solutions will have
Q64: What is the pressure in a gas
Q68: What element is undergoing reduction (if any)
Q77: How many moles of an ideal gas
Q121: Identify what a bomb calorimeter measures.<br>A) measures
Q133: Which of the following have their valence
Q146: Which of the following will cause the
Q150: Identify the number of valence electrons for
Q154: A chemist wishes to calibrate a bomb