Use the standard reaction enthalpies given below to determine 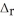 H° for the following reaction: 2S(s) + 3O2(g) → 2SO3(g)
H° for the following reaction: 2S(s) + 3O2(g) → 2SO3(g) 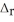 H° = ?
H° = ?
Given:
SO2(g) → S(s) + O2(g) 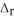 H° = +296.8 kJ
H° = +296.8 kJ
2SO2(g) + O2(g) → 2SO3(g) 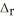 H° = -197.8 kJ
H° = -197.8 kJ
Definitions:
Leadership Co-Created Process Model
A model suggesting leadership is a dynamic and shared process involving leaders and followers collectively contributing to leadership outcomes.
Co-Opt
To assimilate or win over to a larger or established group, usually by offering some form of beneficial association.
Situations
The set of circumstances or contexts in which events occur, affecting the behavior and decisions of individuals and groups.
Practical Perspectives
Considerations or viewpoints focused on real-world applications, effects, or implications rather than theoretical or abstract concepts.
Q26: The density of a gas is 3.12
Q32: valence electrons<br>A)electrons in the outermost shell<br>B)1<br>C)2<br>D)electrons in
Q40: Using the periodic table, identify the element
Q47: To what volume will a sample of
Q81: Identify the major greenhouse gas.<br>A) CO<sub>2</sub><br>B) O<sub>2</sub><br>C)
Q100: What volume of a 0.540 mol L<sup>-1</sup>
Q122: Balance the following equation:<br>_ C<sub>10</sub>H<sub>12</sub> + _
Q155: Calculate the energy of an electron in
Q164: Only two electrons, with opposing spins, are
Q178: Balance the chemical equation given below, and