Use the standard reaction enthalpies given below to determine 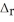 H° for the following reaction: 4SO3(g) → 4S(s) + 6O2(g)
H° for the following reaction: 4SO3(g) → 4S(s) + 6O2(g)  H° = ?
H° = ?
Given:
SO2(g) → S(s) + O2(g) 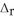 H° = +296.8 kJ
H° = +296.8 kJ
2SO2(g) + O2(g) → 2SO3(g) 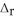 H° = -197.8 kJ
H° = -197.8 kJ
Definitions:
Oligopoly
A market structure characterized by a small number of firms dominating the market, often leading to limited competition.
Monopolistic Competition
A market structure characterized by many firms offering products or services that are similar but not perfect substitutes, leading to each firm having some market power.
Differentiated Products
Goods or services that are distinguished from others based on quality, features, or brand, making them non-identical and often allowing for market power.
Monopolistically Competitive
A market structure where many firms sell products that are similar but not identical, allowing for some degree of market power and product differentiation.
Q8: Determine the concentration of a solution prepared
Q15: -w<br>A)energy flows out of the system into
Q28: The electronegativity is 2.1 for H and
Q45: Why does hot air rise?
Q51: For hydrogen, what is the wavelength of
Q54: How many H<sup>+</sup> ions can the acid
Q100: Use the information provided to determine <img
Q104: Which of the following transitions represent the
Q120: Choose the ground-state electron configuration for Zn<sup>2</sup>⁺.<br>A)
Q171: Automotive air bags inflate when sodium azide