The specific heat capacity of liquid mercury is 0.14 J 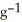
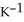 . How many joules of heat are needed to raise the temperature of 4.00 g of mercury from 19.0°C to 39.5°C?
. How many joules of heat are needed to raise the temperature of 4.00 g of mercury from 19.0°C to 39.5°C?
Definitions:
Initial Cash Outlay
The immediate amount of cash required to initiate a project or investment, often including costs such as capital expenditures, setup costs, and any other initial expenses.
Cash Inflows
Money or funds entering a business or project from various sources such as sales, investment, or loans.
Minimax Regret Strategy
A decision-making strategy aimed at minimizing the maximum potential regret for the worst-case scenario outcome.
Opportunity Loss
The loss of potential gain from other alternatives when one alternative is chosen.
Q5: What was the predicted formula for the
Q32: valence electrons<br>A)electrons in the outermost shell<br>B)1<br>C)2<br>D)electrons in
Q88: _ is a piece of equipment designed
Q93: Define constructive interference.
Q100: A 45.0 L steel tank at 20.0
Q127: When forming ionic compounds, cations and anions
Q131: What is the empirical formula for C<sub>4</sub>H<sub>10</sub>O<sub>2</sub>?<br>A)
Q152: A 2.38 g sample of phenol (C<sub>6</sub>H<sub>6</sub>O,
Q155: Calculate the energy of an electron in
Q261: According to the balanced equation shown below,