What is the enthalpy change (in kJ) of a chemical reaction that raises the temperature of 250.0 mL of solution having a density of 1.25 g 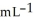 by 10.2 °C? (The specific heat of the solution is 3.733 J
by 10.2 °C? (The specific heat of the solution is 3.733 J 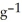
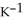 .)
.)
Definitions:
Standard Costs
Predetermined costs for materials, labor, and overhead that are used as benchmarks to measure actual performance and cost control.
Materials Quantity Variance
The difference between the actual quantity of materials used in production and the standard quantity expected to be used, multiplied by the standard cost per unit.
Direct Materials Purchases Variance
The difference between the actual costs of materials purchased and the expected (standard) costs.
Variable Overhead
Costs that fluctuate with changes in production volume, such as utilities or indirect materials, which are part of the overall overhead but vary with the level of output.
Q63: How many molecules of XeF<sub>6 </sub>are formed
Q78: Use the standard reaction enthalpies given below
Q82: Which of the following is a molecular
Q103: Draw the resonance structure that has the
Q114: Choose the best Lewis structure for PO<sub>4</sub><sup>3</sup>⁻.<br>A)
Q124: Draw the best Lewis structure for CH<sub>3</sub><sup>+</sup>.
Q140: What pressure will 2.6 × 10<sup>23</sup> molecules
Q168: Which of the following statements is FALSE?<br>A)
Q221: Calcium oxide reacts with water in a
Q243: Balance the following redox reaction if it