How much heat is absorbed when 50.00 g of C(s) reacts in the presence of excess SO2(g) to produce CS2(l) and CO(g) according to the following chemical equation?
5C(s) + 2SO2(g) → CS2(l) + 4CO(g) 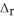 H° = 239.9 kJ
H° = 239.9 kJ
Definitions:
Compulsory Mediation
A mandatory process in certain legal disputes where the parties must attempt to resolve their differences through mediation before proceeding to trial.
Collective Bargaining Legislation
Laws that regulate the negotiation process between employers and a group of employees aimed at establishing working conditions.
Collective Bargaining
The process through which employers and unions negotiate terms of employment.
Elementary Cantonese
A fundamental level of the Cantonese language, involving basic vocabulary and grammar for initial learning stages.
Q55: On the electromagnetic spectrum, visible light is
Q59: Describe the reaction of the alkali metals
Q71: The standard state of carbon is _.
Q89: Which ion does not have a noble
Q110: Which of the following samples will have
Q113: The specific heat capacity of solid copper
Q119: Use the <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7901/.jpg" alt="Use the
Q120: Choose the ground-state electron configuration for Zn<sup>2</sup>⁺.<br>A)
Q129: Why doesn't Dalton's law of partial pressures
Q145: Balance the following redox reaction if it