When 1.50 mol of CH4(g) reacts with excess Cl2(g) at constant pressure according to the chemical equation shown below, 1062 kJ of heat are released. Calculate the value of ΔH for this reaction, as written.
2CH4(g) + 3Cl2(g) → 2CHCl3(l) + 3H2(g) 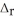 H° = ?
H° = ?
Definitions:
Wrongful Dismissal
Wrongful Dismissal occurs when an employee is terminated from their job in violation of the employment contract or against labor laws protecting employees.
Proper Notice
A legally defined announcement or communication stipulated by law or agreement that is required to be made under specific circumstances.
Damages
Monetary compensation awarded to a party in a lawsuit for loss or injury suffered due to another party's actions or breach of contract.
Reasonable Notice
The sufficient and fair amount of time one party must give another to prepare for or respond to an action, often legally defined in various contexts.
Q17: A gas mixture consists of N<sub>2</sub>, O<sub>2</sub>,
Q20: Give an example of a d orbital.
Q25: Describe the shape of an s orbital.<br>A)
Q27: What volume will a balloon occupy at
Q94: Which of the following substances (with specific
Q100: A 45.0 L steel tank at 20.0
Q104: Use the periodic table to predict the
Q118: A 6.55 g sample of aniline (C<sub>6</sub>H<sub>5</sub>NH<sub>2</sub>,
Q152: A 2.38 g sample of phenol (C<sub>6</sub>H<sub>6</sub>O,
Q260: 7.0 g of nitrogen is reacted with