A fixed amount of gas at 25.0 °C occupies a volume of 20.0 L when the pressure is 0.8386 bar. Use Charles's law to calculate the volume (L) the gas will occupy when the temperature is increased to 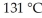 while maintaining the pressure at 0.8386 bar.
while maintaining the pressure at 0.8386 bar.
Definitions:
Weighted Average Cost of Capital
The expected average yield that a company aims to deliver to its security investors for the purpose of funding its assets.
Debt-Equity Ratio
A proportion indicator of how a company leverages debt and equity to finance its asset base.
Market Values
The market's going price for acquiring or selling a service or asset now.
Book Value
The net value of a company's assets minus its liabilities, often used to assess if a stock is under- or overvalued.
Q21: Which of the following occurs as the
Q53: A 14.01 g sample of N<sub>2</sub> reacts
Q60: Use the <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7901/.jpg" alt="Use the
Q80: hydrogen<br>A)Ne(g)<br>B)Cl<sub>2</sub>(g)<br>C)Ca<sub>2</sub>(s)<br>D)Ne<sub>2</sub>(g)<br>E)Ca(s)<br>F)H<sub>2</sub>(g)<br>G)I<sub>2</sub>(s)<br>H)H(g)<br>I)Cl(g)<br>J)O(g)<br>K)O<sub>2</sub>(g)<br>L)I(s)
Q88: _ is a piece of equipment designed
Q116: Identify the colour that has a wavelength
Q143: How many molecules of N<sub>2</sub> are in
Q153: Balance the chemical equation given below, and
Q179: Identify the net ionic equation for the
Q247: Two samples of potassium iodide are decomposed