A solution is prepared by adding 1.40 g of solid NaCl to 50.0 mL of 0.100 mol L-1 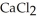 . What is the molarity of chloride ion in the final solution? Assume that the volume of the final solution is 50.0 mL.
. What is the molarity of chloride ion in the final solution? Assume that the volume of the final solution is 50.0 mL.
Definitions:
Chlorination
The addition of chlorine to water or organic compounds, either as a disinfectant or to introduce chlorine atoms into molecules during chemical synthesis.
Methane
A colorless, odorless gas, the simplest alkane and the main component of natural gas; used as a fuel and in organic synthesis.
Termination Reaction
The chemical reaction that concludes a chain reaction process by reacting two or more reactive intermediates to form a stable product.
Chlorination
A chemical process where chlorine is introduced to a compound, often to disinfect or to modify organic molecules in synthesis.
Q20: Use the information provided to determine <img
Q54: Identify what a coffee cup calorimeter measures.<br>A)
Q70: Give the number of core electrons for
Q129: What mass of water, H<sub>2</sub>O<sub> </sub>, contains
Q130: How many grams of calcium hydride are
Q139: Identify the name for PBr<sub>3</sub>.<br>A) phosphorus tribromide<br>B)
Q166: Calculate the atomic mass of element "X"
Q169: Why do the isotopes of the same
Q218: What precipitate is most likely formed from
Q245: Which of the following compounds is an