Lithium and nitrogen react to produce lithium nitride: 6Li(s) + 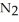 (g) →
(g) → 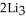 N(s)
N(s)
How many moles of lithium nitride are produced when 0.450 mol of lithium react in this fashion?
Definitions:
Circular Pool
A pool shaped in a perfect circle, often used in geometry to discuss circumference, area, and other circular measurements.
Rectangular Pool
A swimming pool shaped like a rectangle, with two pairs of parallel sides and four right angles.
Divide
A fundamental arithmetic operation that consists of determining how many times one number is contained within another.
Simplify
To reduce a mathematical expression to its most basic form, making it easier to work with.
Q17: The mass number of an atom of
Q27: Combustion analysis of an unknown hydrocarbon produced
Q28: Determine the mass percent (to the hundredths
Q72: How many molecules are in 0.1339 kg
Q73: Calculate the mass of sodium cyanate (NaOCN,
Q112: Determine the mass of water formed when
Q113: How many moles of nitrogen are formed
Q130: When 2.50 g of Ba(s) is added
Q138: +ΔU<br>A)energy flows out of the system into
Q234: What are the required coefficients to properly