Lithium and nitrogen react in a combination reaction to produce lithium nitride: 6Li(s) +  (g) →
(g) → 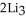 N(s)
N(s)
In a particular experiment, 5.50 g samples of each reagent are reacted. What is the theoretical yield of lithium nitride?
Definitions:
Agglutinogens
Antigens on the surfaces of red blood cells that determine blood types and can cause agglutination.
Agglutinated
The clumping together of particles or cells, often red blood cells by antibodies, typically seen in blood typing tests.
Anti-Rh Antibodies
Immune system proteins that target Rh antigens on red blood cells, important in blood transfusion compatibility and pregnancy complications such as Rh disease.
Transfusion Reaction
An adverse response that occurs when the immune system of a person receiving a blood transfusion attacks the donor blood cells.
Q28: Calculate the final temperature of 68.4 g
Q61: Convert 3000 m h<sup>-1</sup> to km h<sup>-1</sup>.<br>A)
Q83: Determine the density of CO<sub>2</sub> gas at
Q84: Place the following gases in order of
Q101: An 8.21 g sample of glycerol (C<sub>3</sub>H<sub>8</sub>O<sub>3</sub>,
Q103: Consider the following balanced reaction. How many
Q116: What is the correct formula for lead
Q137: Determine the density of an object that
Q155: Identify the number of electrons in P<sup>-3</sup>.<br>A)
Q214: Determine the number of grams H<sub>2</sub> formed