Calcium oxide reacts with water in a combination reaction to produce calcium hydroxide: CaO(s) + 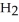 O(l) →
O(l) → 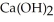 (s)
(s)
A 4.50 g sample of CaO is reacted with 4.34 g of 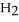 O. How many grams of water remain after the reaction is complete?
O. How many grams of water remain after the reaction is complete?
Definitions:
Arguments
In economics, this often refers to the presentation and examination of reasons or explanations in support of or against economic theories, policies, or practices.
Government Purchases
Expenditures made by the government sector on goods and services to fulfill its various functions and responsibilities.
Transfer Payments
Payments made by governments to individuals without requiring any goods or services in return, such as welfare or social security.
Goods and Services
The output of an economy's business activities, encompassing tangible products (goods) and intangible offerings (services).
Q12: Calculate the change in internal energy (ΔU)
Q23: Which of the following is a molecular
Q47: Identify a substance that is not in
Q88: Determine the volume of SO<sub>2</sub> (at STP)
Q117: Which of the following statements is FALSE
Q119: Identify the name for aqueous H<sub>2</sub>SO<sub>4</sub>.<br>A) sulfuric
Q126: How many moles of CO are contained
Q129: Why doesn't Dalton's law of partial pressures
Q130: How many grams of calcium hydride are
Q209: What volume (in mL) of a 0.0887