Find the inverse of the one-to-one function.
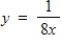
f -1(x) = __________
Definitions:
Hybrid Atomic Orbitals
Orbitals that form from the combination of standard atomic orbitals, explaining the shape and bonding in molecules.
C-C
Refers to the covalent bond between two carbon atoms in organic chemistry.
σ Bond
The strongest type of covalent chemical bond formed by the head-on overlapping of atomic orbitals.
Allene
Organic compounds characterized by a cumulated diene system with two carbon-carbon double bonds.
Q23: Use a calculator to evaluate the expression.(Round
Q32: The vertical distance between the total cost
Q57: Refer to Table 6- 3.If the price
Q67: Refer to Figure 9- 2.If the current
Q135: Use absolute value notation to describe the
Q258: Graphically estimate the x- and y-intercepts of
Q294: Select the correct graph of the given
Q506: Find the average rate of change of
Q556: Find the inverse of the one-to-one function.
Q601: Use a graphing utility to graph the