Multiple Choice
Based on the equations below, which metal is the most active?
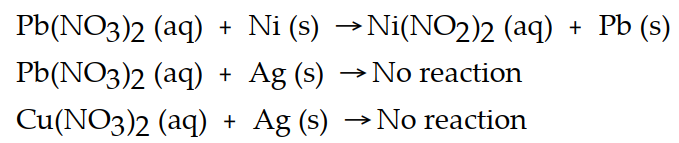
Definitions:
Related Questions
Q6: The balanced equation for the decomposition of
Q28: The have the most negative electron affinities.<br>A)
Q34: The largest principal quantum number in the
Q47: Steel is<br>A) pure iron.<br>B) a liquid at
Q51: Which isoelectronic series is correctly arranged in
Q72: There are mol of bromide ions in
Q107: The secondary structure of a protein is
Q123: Which combination will produce a precipitate?<br>A) AgNO<sub>3
Q125: How many moles of Co<sup>2</sup><sup>+</sup><sup> </sup>are present
Q126: Which group in the periodic table contains