How many moles of magnesium oxide are produced by the reaction of 3.82 g of magnesium nitride with 7.73 g of water? 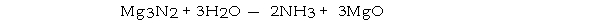
Definitions:
Corporate Experts
Professionals with specialized knowledge and skills in the corporate sector, often providing strategic advice and solutions.
Work Group
A collection of individuals who come together to achieve a common goal or task, often within the context of a workplace or organization.
Organizational Choice
The selection made by individuals when deciding among various organizational opportunities, often influenced by personal values, career objectives, and the organizational culture.
Entry Process
Describes the procedure through which new members are integrated into an organization, including recruitment, selection, and orientation phases.
Q5: The ground state electron configuration of Ga
Q19: Pure acetic acid (HC<sub>2</sub>H<sub>3</sub>O<sub>2</sub>) is a liquid
Q49: Draw a diagram of the short- hand
Q60: The most common sulfur ion has a
Q60: The shape of an orbital is defined
Q76: Which one of the following is false
Q82: The name of CH<sub>3</sub>- CH=C=CH- CH- CH=CH-
Q114: Addition of B<sub>2</sub>O<sub>3 </sub>to soda- lime glass<br>A)
Q147: The ion with the smallest diameter is
Q149: Which is the correct ground- state electron