How many moles of magnesium oxide are produced by the reaction of 3.82 g of magnesium nitride with 7.73 g of water? 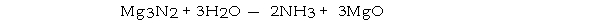
Definitions:
William Lloyd Garrison
A prominent American abolitionist, journalist, and social reformer, known for founding the anti-slavery newspaper "The Liberator."
Frederick Douglass
An American social reformer, abolitionist, orator, writer, and statesman who after escaping from slavery in Maryland, became a national leader of the abolitionist movement.
Elizabeth Cady Stanton
A leading figure in the early women's rights movement, known for her advocacy for women's suffrage, women's rights, and abolition of slavery.
Equivocate
To use ambiguous language so as to conceal the truth or avoid committing oneself to a concrete stance.
Q1: Name the compound, Cu(H<sub>2</sub>O)<sub>4</sub><sup>2</sup><sup>+</sup>.
Q12: A sample of CH<sub>2</sub>F<sub>2</sub><sub> </sub>with a mass
Q31: Of the following compounds, which is the
Q46: Hydrogen is unique among the elements because
Q48: The simplest alkyne is _ .<br>A) ethylene<br>B)
Q49: Isooctane is assigned an octane number of
Q86: The principal quantum number of the first
Q89: Which element is typically not added to
Q101: Which of the following reactions will not
Q140: Which alkali metals can react with oxygen