Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 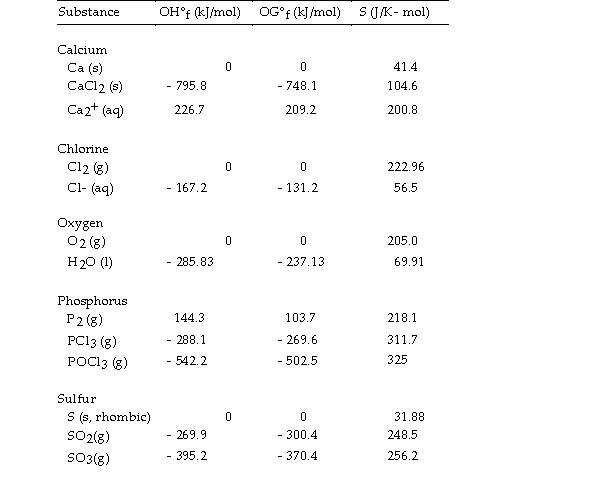
-The value of ΔH° for the decomposition of phosphorous trichloride into its constituent elements,  is kJ/mol.
is kJ/mol.
Definitions:
Sample Size
The number of subjects or units selected from a population to be studied in a statistical sample.
Season Ticket Holders
Individuals or entities that have purchased tickets granting them access to all or a set number of events in a series, typically for sports events or performances, for a particular season.
Standard Normal Distribution
A probability distribution that has a mean of zero and a standard deviation of one, used as the basis for z-scores in statistics.
Margin Of Error
An expression of the amount of random sampling error in a survey's results, representing the extent to which the sample results might differ from those of the actual population.
Q3: The approximate pH of acid rain is
Q18: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1819/.jpg" alt=" The value of
Q30: Which of the following is not a
Q51: Which one of the following statements is
Q54: Calculate OG<sup>○</sup>for the autoionization of water at
Q59: What is the order of the reaction
Q61: In which of the following aqueous solutions
Q77: Of the units below, are appropriate for
Q89: The average rate of disappearance of A
Q91: Carbon can exist in different forms called