Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 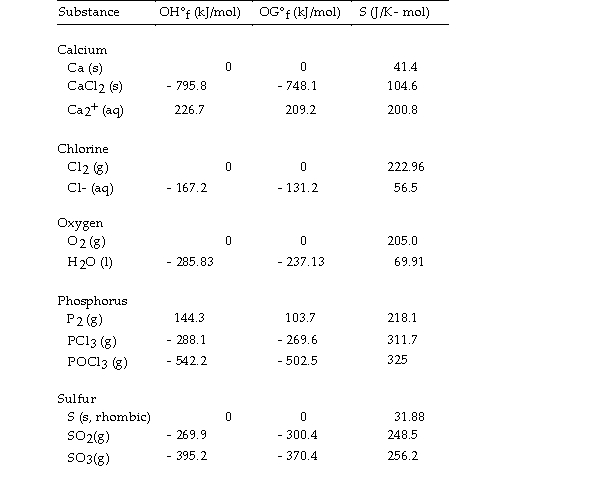
-The value of ΔH° for the decomposition of gaseous sulfur trioxide to its component elements,  is kJ/mol.
is kJ/mol.
Definitions:
Cutting Edge Sources
The most current and innovative resources or information available in a particular field.
Nonmaterial
Pertaining to aspects of a culture or society that are not physical or tangible, such as beliefs, values, rules, and norms.
Social Change
The alteration over time in societal structures and cultural norms and values, which can result from collective human actions, policies, or natural events.
Loosely Defined Norms
Social rules that are not strictly enforced or are vague in nature, often leading to varied interpretations.
Q21: How many moles of B are present
Q48: The equilibrium constant for reaction 1 is
Q52: Cesium- 137 undergoes beta decay and has
Q57: In which of the following aqueous solutions
Q58: What happens in the nucleus of an
Q58: Of the acids in the table below,
Q80: The value of ΔG° at 100.0 °C
Q83: Photoionization processes (e.g., N<sub>2 </sub>+ hv -
Q84: Which of the following reactions will occur
Q201: Which one of the following compounds is