Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 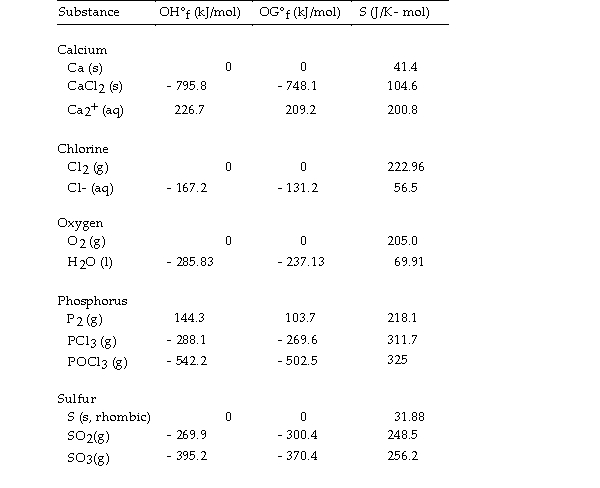
-The value of ΔH° for the decomposition of calcium chloride into its constituent elements,  is kJ/mol.
is kJ/mol.
Definitions:
Discrete Classification
A classification approach that categorizes entities based on clear, distinct boundaries, without overlap between categories.
Categorical Classification
A method of organizing items or concepts into distinct categories based on specific criteria or attributes.
Dimensional Classification
Dimensional Classification is an approach in psychology that categorizes mental disorders based on a continuum of severity rather than discrete categories, considering the degree of symptoms.
Medical Model
A framework in which psychological or behavioral problems are seen as symptoms of underlying physical issues or diseases, and treated through medical interventions.
Q16: The more the value of E°<sub>red</sub>, the
Q18: At constant temperature, reducing the volume of
Q20: A BrØnsted- Lowry base is defined as
Q53: An aqueous solution contains 0.050 M of
Q62: Phosphorous and chlorine gases combine to produce
Q70: What is the pH of an aqueous
Q71: CO<sub>2</sub><sub> </sub>from hydrocarbon combustion creates a major
Q92: H<sub>2</sub>SeO<sub>4 </sub>is called selenic acid.
Q96: A second- order reaction has a half-
Q119: The mass of a proton is 1.00728