Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 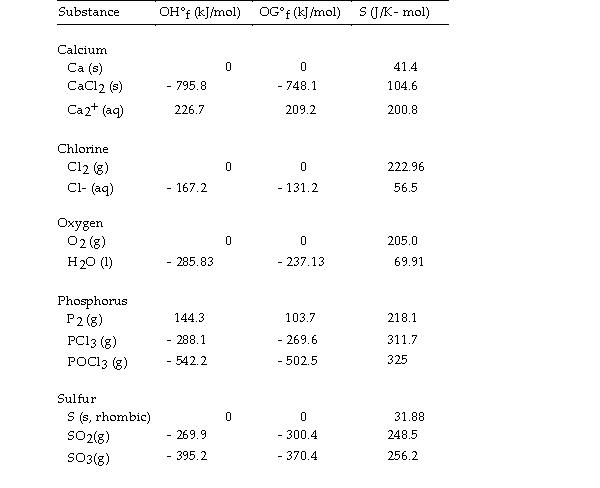
-The value of ΔG° at 25 °C for the oxidation of solid elemental sulfur to gaseous sulfur trioxide,  is kJ/mol.
is kJ/mol.
Definitions:
Post-Closing Trial Balance
A list of all accounts still open after closing entries are made, used to ensure that debits equal credits.
Capital
The wealth in the form of money or other assets owned by a person or organization, or available for a purpose such as starting a company or investing.
Purchases
The total cost of goods acquired by a company for resale or use in production during an accounting period.
Freight-In Account
An accounting term for the cost of transporting goods into a business, which is part of the cost of goods sold.
Q7: The average adult in the U.S. needs
Q8: An acid containing the COOH group is
Q10: For the elementary reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1819/.jpg" alt="For
Q13: How many neutrons are emitted when a
Q28: The contribution of sulfur to acid rain
Q31: How many minutes will it take to
Q48: Galvanized iron is iron coated with .<br>A)
Q77: How many radioactive decay series exist in
Q100: Natural, unpolluted rainwater is typically acidic. What
Q105: What is a phosphor?<br>A) a bioluminescent substance<br>B)