Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 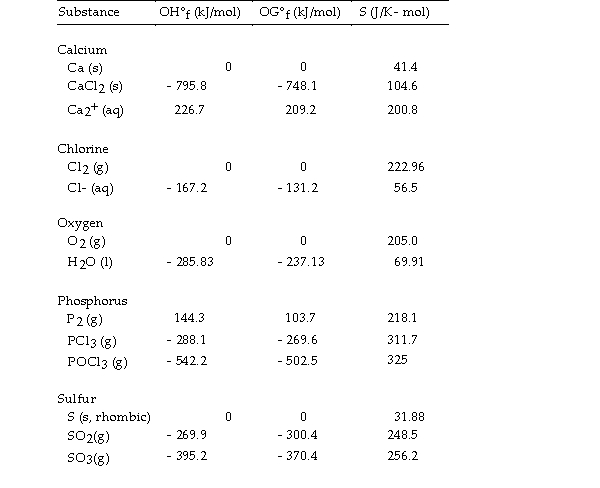
-The value of ΔS° for the decomposition of calcium chloride into its constituent elements,  is J/K.
is J/K.
Definitions:
Metacognition
The awareness and understanding of one's own thought processes.
Information-Processing Capacity
The extent to which an individual can attend to, store, and manage information to perform tasks efficiently and effectively.
Moral Considerations
Factors related to the principles of right and wrong behavior guiding decision-making.
Proposition
The meaning or content of a statement.
Q7: The average adult in the U.S. needs
Q23: Calculate the pH of a solution that
Q35: The rate of a reaction depends on
Q40: What is the formula of the compound
Q40: What is emitted in the nuclear transmutation,<img
Q66: Which substance is the reducing agent in
Q74: The acid- dissociation constant at 25.0 °C
Q87: In the nuclear transmutation represented by <img
Q121: Strontium- 90 is a byproduct in nuclear
Q150: Isotopes are atoms that have the same