Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 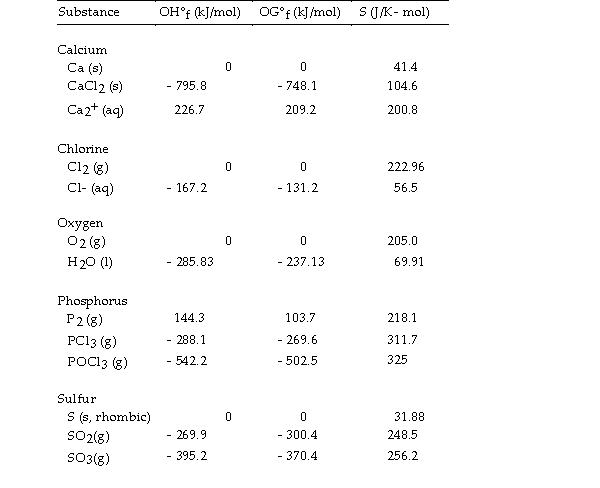
-The value of ΔG° at 25 oC for the decomposition of gaseous sulfur trioxide to solid elemental sulfur and gaseous oxygen,  is kJ/mol.
is kJ/mol.
Definitions:
Accrual Basis
An accounting method where revenues and expenses are recorded when they are earned or incurred, regardless of when cash is exchanged.
Direct Method
A way of reporting cash flows from operating activities by showing major classes of gross cash receipts and payments.
Operating Expenses
Costs associated with the day-to-day operations of a business, excluding direct material and labor costs.
Q12: A 25.0 mL sample of 0.150 M
Q24: The molar concentration of hydroxide ion in
Q25: Silver has two naturally occurring isotopes with
Q45: In the nuclear transmutation represented by <img
Q55: The primary buffer system that controls the
Q69: The enzyme nitrogenase converts _ _ into
Q77: A 25.0 mL sample of 0.723 M
Q89: Of the three types of radioactivity characterized
Q102: The kinetics of the reaction below were
Q163: the possible oxication numbers for gold are