Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 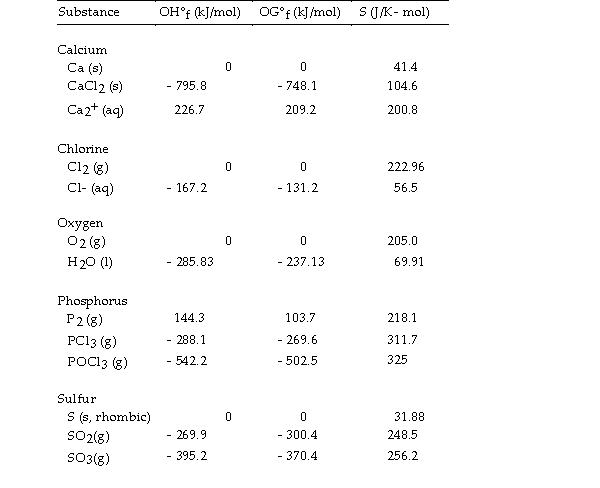
-The value of ΔS° for the decomposition of gaseous sulfur trioxide to solid elemental sulfur and gaseous oxygen,  is J/K.
is J/K.
Definitions:
Equilibrium
A state of balance or stability within a system, where opposing forces or influences are in equal proportion.
Change
The process through which something becomes different, often leading to new ways of thinking, behaving, or operating.
Enlightenment
A philosophical movement of the 18th century emphasizing individualism, reason, and skepticism towards traditional doctrines.
Natural Laws
A body of unchanging moral principles regarded as a basis for all human conduct.
Q1: The Henry's law constant for helium gas
Q29: A freshly prepared sample of curium- 243
Q38: The solubility of lead (II) chloride (PbCl<sub>2</sub>)
Q40: A 0.100 m solution of which one
Q42: A solution of acetic acid is 2.0%
Q51: _discovered radioactivity.
Q58: Find the temperature above which a reaction
Q59: In balancing the nuclear reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1819/.jpg"
Q71: Consider the reaction:<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1819/.jpg" alt="Consider the reaction:
Q99: The mass of a proton is 1.673