Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 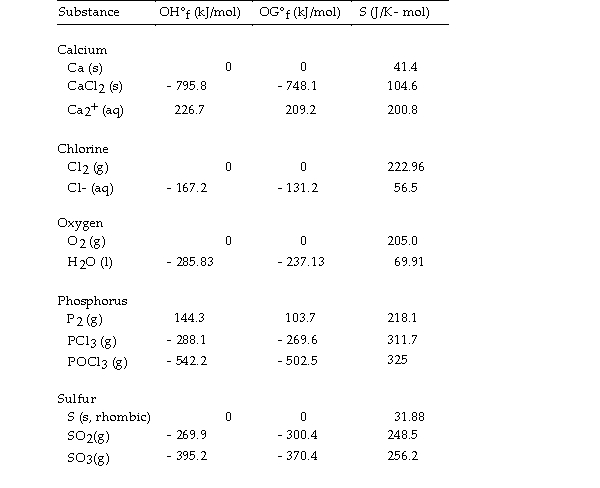
-The value of ΔS° for the decomposition of phosphorous trichloride into its constituent elements,  is J/K.
is J/K.
Definitions:
Miller-Orr Model
A financial management model that helps firms manage cash balances by setting upper and lower limits on cash reserves.
Optimal Upper Cash Limit
The optimal upper cash limit is the maximum amount of cash a company determines it should hold to efficiently manage transactions and emergencies while minimizing holding costs.
Standard Deviation
A statistical measure of the dispersion or variability in a set of values, often used in finance to quantify the risk of an investment's return.
Net Present Value
The gap between the current value of incoming and outgoing cash over a given period.
Q1: The mole fraction of neon in dry
Q18: The name of the ionic compound V<sub>2</sub>O<sub>3</sub><sub>
Q29: A 25.0 mL sample of a solution
Q43: Which substance in the reaction below either
Q52: The value of ΔG° at 25 °C
Q61: In which of the following aqueous solutions
Q85: What is the oxidation number of potassium
Q90: What was the purpose of the Manhattan
Q113: The correct name for H<sub>2</sub>SO<sub>3</sub><sub> </sub>is _
Q144: What is the molecular formula for heptane