Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 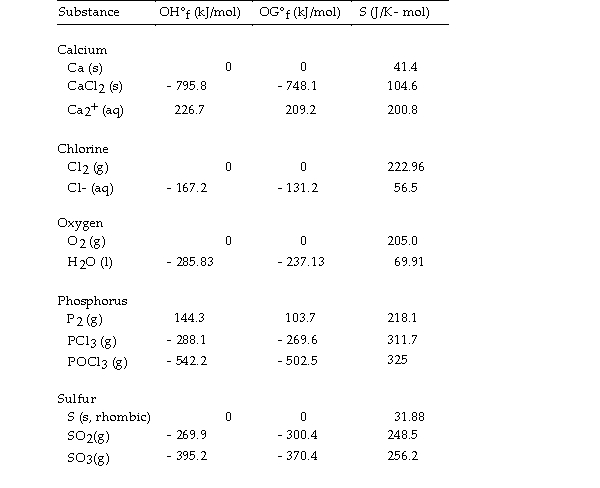
-The value of ΔH° for the decomposition of gaseous sulfur dioxide to solid elemental sulfur and gaseous oxygen,  is kJ/mol.
is kJ/mol.
Definitions:
Accommodation
The ability of the eye to change its focus from distant to near objects, involving the lens changing shape.
Constricts
To narrow or become tighter, often referring to the narrowing of blood vessels or other tubular structures.
Accommodation Reflex
The process by which the eye changes optical power to maintain a clear image or focus on an object as its distance varies.
Photopupillary Reflex
The reflex constriction of the pupils when the eyes are exposed to bright light.
Q6: The formula of bromic acid is _.<br>A)
Q18: What is the oxidation number of manganese
Q18: The mechanism for formation of the product
Q19: The overall reaction order is the sum
Q19: Which one of the following is a
Q21: Consider a solution containing 0.100 M fluoride
Q50: The overall order of a reaction is
Q91: Carbon can exist in different forms called
Q102: The kinetics of the reaction below were
Q138: Which formula/name pair is incorrect? <br>A) <img