Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 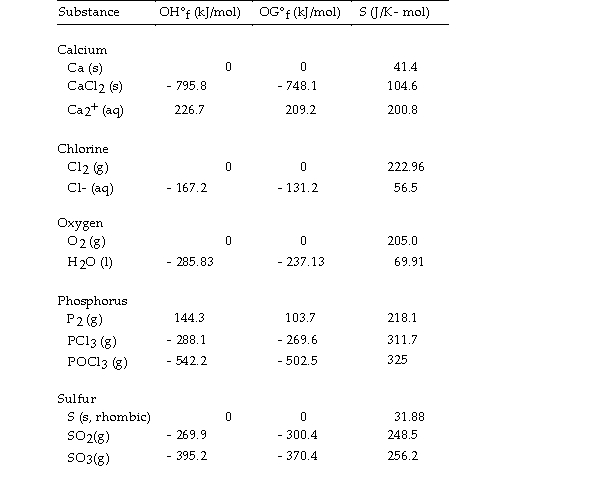
-The value of ΔG° at 373 °K for the oxidation of solid elemental sulfur to gaseous sulfur dioxide,  is kJ/mol. At 298K, OH° for this reaction is - 269.9 kJ/mol, and OS° is +11.6 J/K.
is kJ/mol. At 298K, OH° for this reaction is - 269.9 kJ/mol, and OS° is +11.6 J/K.
Definitions:
High-Performance Climates
Environments within organizations that foster exceptional performance levels through motivating and engaging employees.
Dynamic Environment
A situation or context that is constantly changing, often unpredictably, requiring flexibility and quick adaptation.
Competitive Pressures
The challenges and forces that businesses face from rivals in the market, pushing them to improve, innovate, and efficiently manage resources.
Information Technology
The use of computers, storage, networks, and other physical devices, infrastructure, and processes to create, process, store, secure, and exchange all forms of electronic data.
Q5: Of the following, the entropy of gaseous
Q24: An unsaturated solution is one that _.<br>A)
Q24: Calculate the pH of a solution prepared
Q32: The magnitude of K<sub>w</sub><sub> </sub>indicates that .<br>A)
Q34: The more negative OG<sup>○</sup>is for a given
Q40: In which aqueous system is PbI<sub>2 </sub>least
Q40: What is emitted in the nuclear transmutation,<img
Q45: Clean rainwater is acidic mainly due to
Q54: Why does fluoride treatment render teeth more
Q150: Isotopes are atoms that have the same