Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 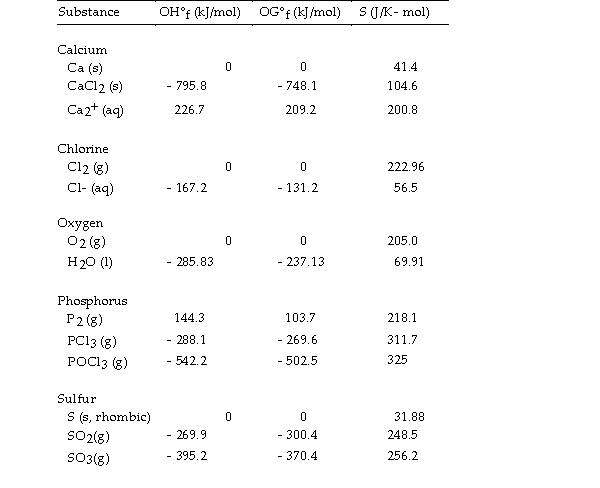
-The value of ΔS° for the formation of calcium chloride from its constituent elements,  is J/K.
is J/K.
Definitions:
Set of Scores
A collection of numerical values that represent observations or measurements.
Central Tendency
Statistical measures that identify a single value as representative of the middle or center of a distribution, including mean, median, and mode.
Data
Facts and statistics collected together for analysis or used to reason or make decisions.
Weighted Mean
An average where each data point contributes proportionally to the total based on its assigned weight, reflecting its importance.
Q11: The equilibrium- constant expression depends on the
Q19: The acid- dissociation constants of sulfurous acid
Q21: How many grams of Ca metal are
Q27: Of the following, which is the strongest
Q30: Which of the following is not a
Q33: The mass of a proton is 1.00728
Q38: A reaction that is not spontaneous at
Q73: Which pair of elements would you expect
Q96: A second- order reaction has a half-
Q115: Potassium forms an ion with a charge