The value of ΔG° at 141.0 °C for the formation of phosphorous trichloride from its constituent elements, 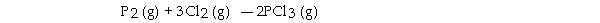 is kJ/mol. At 25.0 oC for this reaction, ΔH° is - 720.5 kJ/mol, ΔG° is - 642.9 kJ/mol, and
is kJ/mol. At 25.0 oC for this reaction, ΔH° is - 720.5 kJ/mol, ΔG° is - 642.9 kJ/mol, and
ΔS° is - 263.7 J/K.
Definitions:
Ending Inventory
is the value of goods available for sale at the end of an accounting period, calculated by adding new purchases to the starting inventory, then subtracting the cost of goods sold.
Budgeted Sales
Projected or planned sales for a future period, often used for planning and financial forecasting.
Master Budget
A comprehensive financial plan for an organization, including all individual budgets related to sales, production, overheads, etc., for a specific period.
Direct Labor
The wages and salaries for the workers who are directly involved in the production of goods, easily traceable to the product.
Q3: Which below best describe(s) the behavior of
Q27: Which substance is serving as the reducing
Q40: In which aqueous system is PbI<sub>2 </sub>least
Q41: The only element with no neutrons is
Q52: Which of the following is arranged correctly
Q59: In balancing the nuclear reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1819/.jpg"
Q74: The energy produced by the sun is
Q87: A solution is prepared by dissolving 0.23
Q95: Which statement below correctly describes the responses
Q97: Calcium forms an ion with a charge