Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 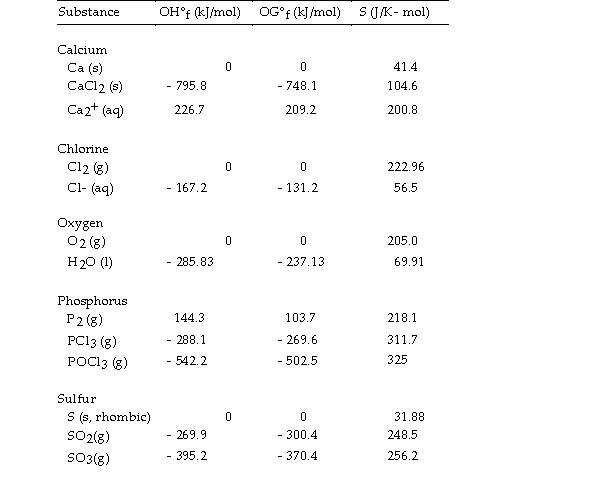
-The value of ΔH° for the decomposition of phosphorous trichloride into its constituent elements,  is kJ/mol.
is kJ/mol.
Definitions:
Market Segments
The process of dividing a broad target market into smaller, more homogeneous groups of customers with similar needs, characteristics, or behaviors.
Consumer Price Points
Predetermined prices at which customers are willing to buy a product or service.
Inter-consumer Communication
The exchange of information, perceptions, and opinions between customers, often influencing purchasing decisions and brand perceptions.
Innovative New Products
Refers to products that introduce unique features, benefits, or technological advancements, distinguishing them from existing offerings in the market.
Q6: What is the coefficient of the permanganate
Q16: The more the value of E°<sub>red</sub>, the
Q19: Which one of the following is a
Q25: The combustion of ethylene proceeds by the
Q58: Find the temperature above which a reaction
Q62: The reaction<br>2NO<sub>2 </sub>→ 2NO + O<sub>2</sub><br>Follows second-
Q78: The base- dissociation constant of ethylamine (C<sub>2</sub>H<sub>5</sub>NH<sub>2</sub>)
Q88: Which one of the following is most
Q100: The reaction A (aq) - B (aq)
Q141: The nucleus of an atom does not