Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 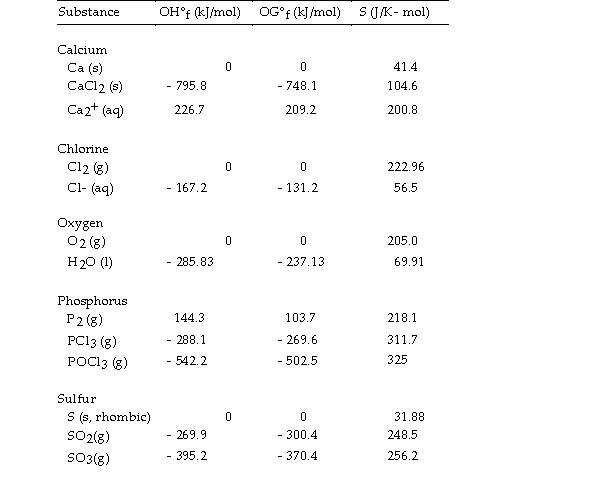
-The value of ΔG°at 25 °C for the decomposition of phosphorous trichloride into its constituent elements, 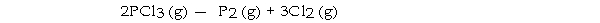 is kJ/mol.
is kJ/mol.
Definitions:
Social Media
Platforms and websites that enable users to create and share content or participate in social networking.
Ultimate Consumers
The final buyers or users of products and services for personal, non-business use.
Promotional Mix
The combination of advertising, personal selling, sales promotion, public relations, and direct marketing methods used to reach target customers and achieve marketing goals.
Pull Strategy
A marketing strategy designed to create demand for products directly from consumers, leading retailers to stock the product due to increased consumer demand.
Q24: SO<sub>2</sub>Cl<sub>2</sub><sub> </sub>decomposes in the gas phase by
Q25: If we start with 1.000 g of
Q28: The anode of the alkaline battery is
Q29: What is the pH of an aqueous
Q30: The elementary reaction<br>2NO<sub>2 </sub>(g) → 2NO (g)
Q47: The thermodynamic quantity that expresses the degree
Q60: The layer of the atmosphere that contains
Q78: Which substance is the reducing agent in
Q107: Radioactive decay is a first order kinetic
Q110: Units of the rate constant of a