Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 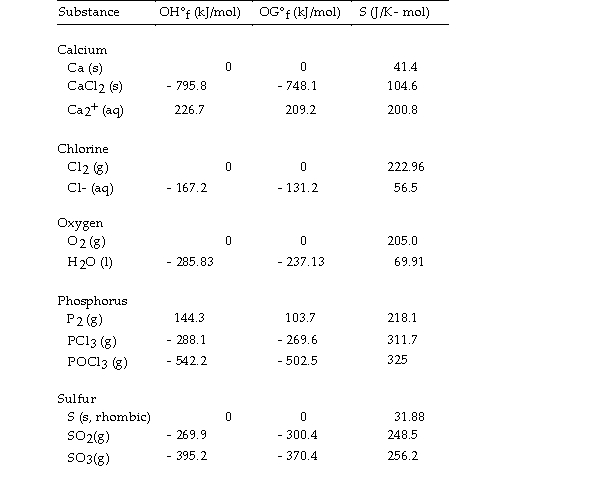
-The value of ΔH° for the decomposition of gaseous sulfur trioxide to its component elements,  is kJ/mol.
is kJ/mol.
Definitions:
Racialization
The process by which society ascribes racial identities to a particular group and then imbues those meanings with social, economic, and political significance.
Police Searches
Inspections conducted by law enforcement officials to find evidence of criminal activity.
White-collar Crime
Non-violent crime committed by individuals or corporations in the course of their professional lives, often involving financial deception.
Media Mogul
A highly influential person in the media industry who owns, controls, or has a significant impact on large segments of the mass media.
Q20: A molecular formula always indicates .<br>A) the
Q31: Oxygen is a _ and nitrogen is
Q39: The main scientific difficulty in achieving a
Q60: How many kilowatt- hours of electricity are
Q62: What is the formula for perchloric acid?<br>A)
Q66: A substance that is capable of acting
Q68: Consider an electrochemical cell based on the
Q71: The kinetics of the reaction below were
Q159: Which isotope has 36 electrons in an
Q177: Which one of the following compounds is