Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 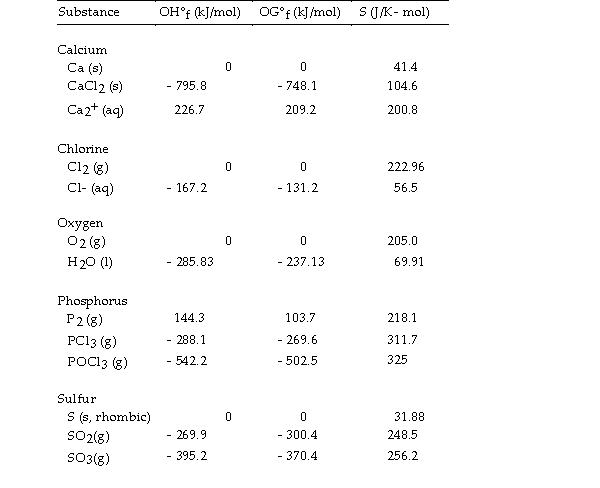
-The value of ΔG° at 25 oC for the decomposition of gaseous sulfur trioxide to solid elemental sulfur and gaseous oxygen,  is kJ/mol.
is kJ/mol.
Definitions:
Bonds Payable
Long-term liabilities represented by bonds that a company must repay at a future date, often including periodic interest payments.
Cash Dividend
A distribution of profits by a company to its shareholders, typically given as cash.
Direct Method
An approach in cost accounting where specific costs are directly traced to cost objects without any allocation, ensuring clear cost attribution.
Operating Activities
Activities related to the day-to-day functioning of a business, including selling, administering, and producing goods and services.
Q3: In a lead- acid battery, the electrodes
Q9: An unknown element is found to have
Q11: The hydride ion, H<sup>-</sup><sup> </sup>, is a
Q15: What is the anode in an alkaline
Q44: The standard emf for the cell using
Q47: The thermodynamic quantity that expresses the degree
Q72: The lithium ion battery has more energy
Q73: The electode where reduction occurs is called
Q91: The base- dissociation constant, K<sub>b</sub>, for pyridine,
Q192: Elements in Group 2A are known as