Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 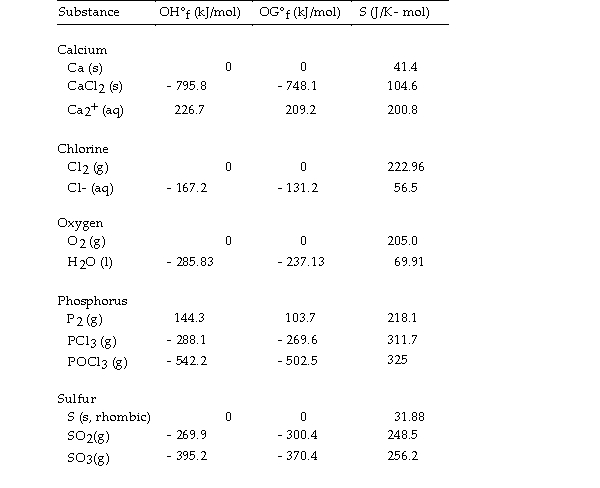
-The value of ΔS° for the decomposition of phosphorous trichloride into its constituent elements,  is J/K.
is J/K.
Definitions:
Sampling
The process of selecting a subset of individuals or items from a larger population to estimate characteristics of the whole population.
Hawthorne Studies
A series of studies conducted in the early 20th century that examined how various factors in the workplace affected worker productivity, leading to insights in organizational psychology.
Human Resource Strategy
A long-term plan for managing and optimizing an organization's workforce in alignment with its overall goals.
Job Design
The process of defining how work will be performed and what tasks will be required in a given job to achieve efficiency, quality, and satisfaction for both the organization and the employee.
Q22: The mass number of an atom of
Q43: Of the following, is a weak acid.<br>A)
Q48: <sup>131</sup>I has a half- life of 8.04
Q57: The primary detrimental effect of the presence
Q76: There are protons, neutrons, and electrons in
Q109: The rate law for a reaction is<br>Rate
Q123: Magnesium reacts with a certain element to
Q147: Which pair of elements is most apt
Q154: The correct name for HClO<sub>3</sub><sub> </sub>is _
Q158: What is the molecular formula for propane