Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 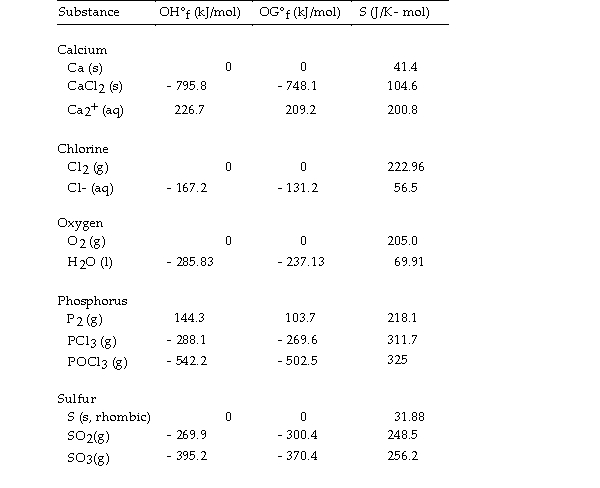
-The value of ΔH° for the decomposition of gaseous sulfur dioxide to solid elemental sulfur and gaseous oxygen,  is kJ/mol.
is kJ/mol.
Definitions:
Demand for Tea
The quantity of tea that consumers are willing and able to purchase at various prices within a given time period.
Demand Curve
A graph showing the relationship between the price of a good and the quantity of the good that buyers are willing to purchase at various prices.
Miami Dolphins
An American professional football team based in the Miami metropolitan area that competes in the National Football League (NFL).
Season Tickets
Long-term passes offered at a reduced rate, granting the holder access to a series of events or entry to a venue.
Q22: Using the data in the table, which
Q57: The relative biological effectiveness (RB<br>E) is 10
Q59: The K<sub>a </sub>of hypochlorous acid (HClO) is
Q59: The concentration of carbon monoxide in a
Q61: In which of the following aqueous solutions
Q68: The gold foil experiment performed in Rutherford's
Q87: The half- life of a first- order
Q96: The entropy of the universe is .<br>A)
Q125: In the absence of magnetic or electric
Q166: The charge on the manganese in the