Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 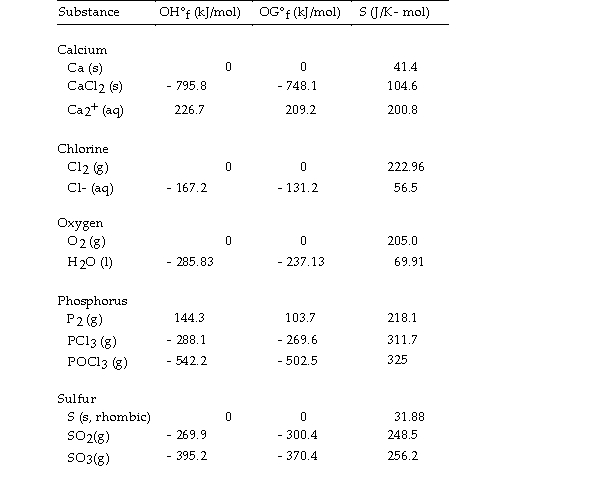
-The value of ΔG° at 373 °K for the oxidation of solid elemental sulfur to gaseous sulfur dioxide,  is kJ/mol. At 298K, OH° for this reaction is - 269.9 kJ/mol, and OS° is +11.6 J/K.
is kJ/mol. At 298K, OH° for this reaction is - 269.9 kJ/mol, and OS° is +11.6 J/K.
Definitions:
Board of Directors
The board of directors is a group of individuals elected to represent shareholders and oversee the activities and strategic direction of a company.
CPA Canada Handbook
A comprehensive guide that provides the accounting and assurance standards for Canadian accountants, serving as a crucial resource for financial reporting.
Private Not-for-Profit Organizations
Organizations that operate for the public good without the intention of making profits for owners or shareholders.
ASPE
A framework of accounting guidelines tailored for privately owned Canadian entities to maintain consistency in financial reporting.
Q5: The average rate of disappearance of A
Q29: The relationship of absorbed light to the
Q30: The elementary reaction<br>2NO<sub>2 </sub>(g) → 2NO (g)
Q47: Calculate the pH of a solution prepared
Q51: Which one of the following statements is
Q69: The enzyme nitrogenase converts _ _ into
Q74: A voltaic cell is constructed with two
Q81: Rates of reaction can be positive or
Q99: Iodine forms an ion with a charge
Q137: The correct name for CaH<sub>2</sub><sub> </sub>is _