Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 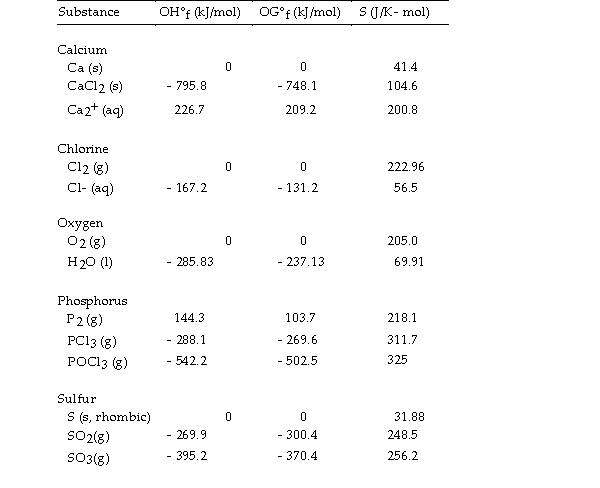
-The value of ΔS° for the formation of calcium chloride from its constituent elements,  is J/K.
is J/K.
Definitions:
Probable Event
An event that is likely to occur based on current evidence or past experience.
Estimable Amount
A quantifiable sum that can be accurately predicted or approximated in the accounting records.
Interest Calculations
The process of determining the interest payment on a loan or investment, based on the principal, rate, and time.
Merchandise
Merchandise consists of goods that a retailer purchases to sell to its customers for profit.
Q1: A 25.0 mL sample of an HCl
Q18: At constant temperature, reducing the volume of
Q43: What are the principal organs that regulate
Q50: Charged particles are accelerated because the faster
Q56: The concentration of A is M after
Q63: A system that doesn't exchange matter or
Q80: Of the following processes, which one changes
Q94: Of the three types of radioactivity characterized
Q100: Natural, unpolluted rainwater is typically acidic. What
Q164: Which one of the following species has