Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 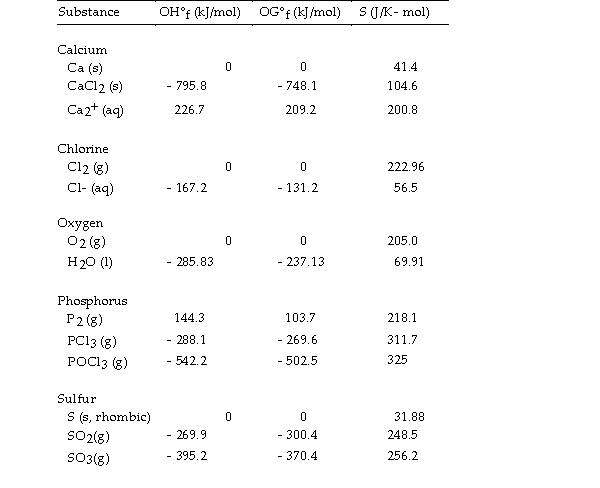
-The value of ΔG° at 373 K for the oxidation of solid elemental sulfur to gaseous sulfur trioxide,  is kJ/mol.
is kJ/mol.
Definitions:
Simultaneous Game
A strategic situation where each player must make their decisions at the same time, without knowledge of the other players' choices.
Positive-Sum Game
A situation in which all participants can gain or benefit in some way, contrasting with a zero-sum game.
Zero-Sum Game
A situation in economics or game theory where one party's gain is precisely balanced by another party's loss.
Dominant Strategy
In game theory, a strategy that is best for a player regardless of the strategies chosen by the other players.
Q5: Biodegradable material degraded by aerobic processes ends
Q20: In which of the following reactions would
Q22: In the formula k=0.693/t<sub>1/2</sub>, k is the
Q29: The phrase "like dissolves like" refers to
Q38: Of the following, only does not result
Q39: What current (in a) is required to
Q43: Consider the following equilibrium.<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1819/.jpg" alt="Consider the
Q44: Different isotopes of a particular element contain
Q60: There are electrons, protons, and neutrons in
Q64: A 0.15 M aqueous solution of the