Consider the following reaction at equilibrium: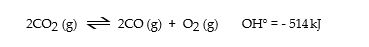
Le Cha^telier's principle predicts that adding O2 (g) to the reaction container will _ .
Definitions:
Interest Earning
Refers to the process by which money accrues interest over time in a savings account or investment.
Bank Account
A financial account maintained by a banking institution where a customer can deposit and withdraw money.
Simple Rate
A simple interest rate is calculated only on the principal amount of a loan or investment, without compounding over time.
Interest Charged
The amount of money charged by a lender to a borrower for the use of borrowed funds, expressed as a percentage of the principal.
Q7: A solution of NaF is added dropwise
Q21: The dividing line between the troposphere and
Q22: If ΔG° for a reaction is greater
Q62: Of the following substances, only has London
Q69: According to the Arrhenius concept, an acid
Q70: The concentration of sodium chloride in an
Q80: The magnitude of the rate constant is
Q81: The pH of a 0.10 M solution
Q94: What is the final stage in municipal
Q107: Which of the following substances is more