What is a possible set of quantum numbers for an unpaired electron in the orbital box diagram below? 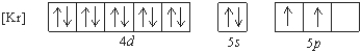
Definitions:
Applied Research
Research meant to provide practical solutions to immediate problems.
Developmentalists
Specialists who study the development of individuals across their lifespan, often focusing on childhood development.
Lifespan
The duration of time from an individual's birth to their death, also studied as the developmental stages and changes experienced throughout this period.
Theoretical Perspective
A framework or approach to understanding and explaining various phenomena within a certain field of study.
Q8: An excess of sodium hydroxide is treated
Q8: Refer to diagram 9-1. Identify the molecule
Q12: Which of the following elements would be
Q21: All of the following ground-state electron configurations
Q47: Which of the following sets of
Q58: Which of the following elements has the
Q60: How many sigma (σ) bonds and pi
Q75: The percent yield of a chemical reaction
Q78: Naturally occurring element X exists in three
Q80: Which of the following statements is true?<br>A)