What is a possible set of quantum numbers for an unpaired electron in the orbital box diagram below? 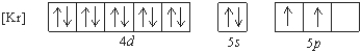
Definitions:
Detailed Insights
involves in-depth analysis and comprehensive understanding of specific data, trends, or phenomena.
Herfindahl Index
A tool for measuring business sizes within their respective sectors, providing insight into the competitive landscape.
Four-firm Concentration Ratio
A measure that represents the market share of the four largest firms in an industry, used to assess the degree of competition and concentration in the market.
Mutual Interdependence
A situation in which entities rely on each other to a significant extent, particularly relevant in markets with a few dominant firms.
Q11: A gaseous mixture containing 1.5 mol Ar
Q27: Which of the following is the correct
Q27: Convert 1.400× 10<sup>-8</sup> liters to nanoliters and
Q41: The (principal quantum number) n = _
Q50: The figure below depicts a shooting target.
Q51: Arrange the following cations in the increasing
Q54: A sample of helium gas occupies 17.9
Q58: Which anion will form a precipitate with
Q66: Non-ideal behavior for a gas is most
Q74: Use Lewis structures to predict the bond