When 1 mole of Fe2O3(s) reacts with H2(g) to form Fe(s) and H2O(g) by the following reaction, 98.8 kJ of energy is absorbed.
Fe2O3(s) + 3 H2(g) → 2 Fe(s) + 3 H2O(g) 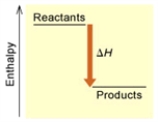 (A)
(A) 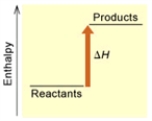
(B)
Is the reaction endothermic or exothermic, and which of the enthalpy diagrams above
Represents this reaction?
Definitions:
Future Cash Flows
These are the estimated amounts of money expected to be received or paid out in the future as a result of current investments, operations, or financial decisions.
Pro Forma Financial Statements
Financial statements that project the future financial position of a company based on current data and assumptions about future events.
Worst-Case Scenarios
The most adverse, yet possible, outcomes that can occur under certain conditions.
Competitor Behavior
Actions and strategies employed by businesses in the same industry that influence market dynamics and competitive landscapes.
Q7: For a proton (mass = 1.673 ×
Q12: An intensive property of a substance is<br>A)
Q14: Atoms are made of three subatomic particles.
Q36: The correct name for Cd<sup>2+</sup> is _.<br>A)
Q47: A 2.288 g sample of a hydrocarbon
Q57: Which molecule will have the following valence
Q64: List all the intermolecular forces present in
Q65: What charge is likely possible on a
Q156: The major disadvantage of using forced tube-feeding
Q171: Depression and eating disorders are correlated.What does