Refer to the graph below. 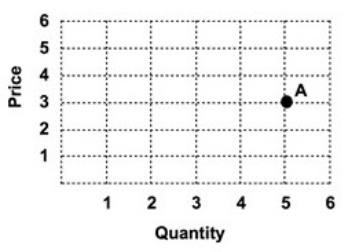 Point A represents a price of:
Point A represents a price of:
Definitions:
Homogeneous Catalyst
A catalyst that exists in the same phase (solid, liquid, or gas) as the reactants in a chemical reaction, promoting the reaction without being consumed.
Zero-order Reaction
A chemical reaction where the speed at which it occurs is unaffected by the amount of reactant(s) present.
Reactant Concentration
The amount of reactants present in a given volume of solution at the start of a chemical reaction, influencing the reaction rate.
First-order Reaction
A chemical reaction whose rate is directly proportional to the concentration of a single reactant.
Q4: Describe the contributions of the Environmental Defense
Q11: A precept is:<br>A) the application of models
Q17: Herman is a graphic designer at a
Q38: Mali is a lower-level employee of extreme
Q41: Using the classification system of John French
Q42: Businesses produce goods and services and sell
Q64: Refer to the graph showing the demand
Q74: Economic forces:<br>A) are a reaction to scarcity.<br>B)
Q128: Refer to the table shown that
Q133: Which of the following is one of