What is wrong with the Lewis structure shown for sulfur trioxide,SO3? 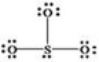
Definitions:
Telecommuting Program
A work arrangement that allows employees to perform their job duties from outside the traditional office environment, often from home, facilitated by technological tools.
Satellite Office
denotes a secondary office located away from a company's main office, used to accommodate remote employees or serve different geographical areas.
Compressed Workweek
A flexible work schedule allowing employees to work longer hours on some days and fewer on others, completing a full workweek in fewer days.
Core Time
The period during a work schedule when all employees are required to be present, often used in flexible working arrangements.
Q1: All atoms of a particular element have
Q20: Which statement concerning the stability of nuclei
Q43: An alloy,such as brass,is an example of
Q49: Which statement concerning the classification of matter
Q56: For the reaction 2H<sub>2</sub>(g)+ S<sub>2</sub>(g)⇌ 2H<sub>2</sub>S(g),determine the
Q64: Resonance occurs when two or more different,valid
Q72: Which compound(s)below contain substituents that are cis?
Q74: How many atoms of sulfur are present
Q79: What type of radioactive decay is
Q101: Round 0.052018 to three significant figures.<br>A)0.05<br>B)0.052<br>C)0.0520<br>D)0.05201<br>E)0.05202