What is wrong with the Lewis structure shown for sulfur trioxide,SO3? 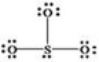
Definitions:
Behavioral Interview
A structured interview that is used to collect information about past behavior.
Structured Interview
A systematic interviewing method where each interviewee is asked the same set of predetermined questions in the same order.
Past Behavior
Refers to an individual's previous actions or conduct, often considered in psychological assessments or predictive models of future behavior.
Achenbach System
A standardized tool used for assessing emotional and behavioral problems in children and adolescents.
Q13: Which of the following would NOT describe
Q13: Howard received $12 000 of the $39
Q25: Assuming that air is a solution containing
Q28: How many valence electrons are present in
Q41: What does the prefix "centi-" mean?<br>A)10<sup>-1</sup><br>B)10<sup>-2</sup><br>C)10<sup>-3</sup><br>D)10<sup>2</sup><br>E)10<sup>3</sup>
Q61: The concentration of a patient's blood sugar
Q63: Iron reacts with oxygen to form iron(III)oxide
Q69: Assuming reactions between the following pairs of
Q69: What property of liquids explains the formation
Q75: An entire contract clause could be used